First Law Of Thermodynamics Definition Chemistry
Thermodynamics thermodynamics the first law of thermodynamics.
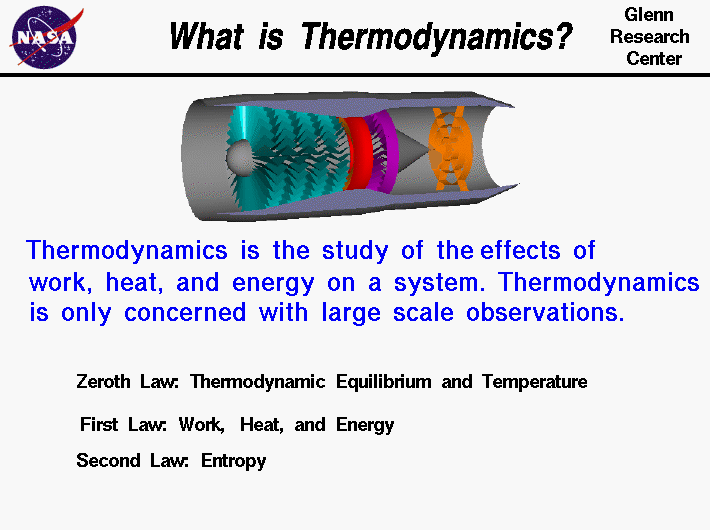
First law of thermodynamics definition chemistry. In a closed system i e. The first law of thermodynamics. Second law of thermodynamics. The first law of thermodynamics is the physical law which states that the total energy of a system and its surroundings remain constant.
There are four laws which govern the thermodynamic systems phenomena they are. Or the total energy of a system and its surrounding remain constant. Energy can be transformed from one form to another but can be neither created nor destroyed. The first law of thermodynamics.
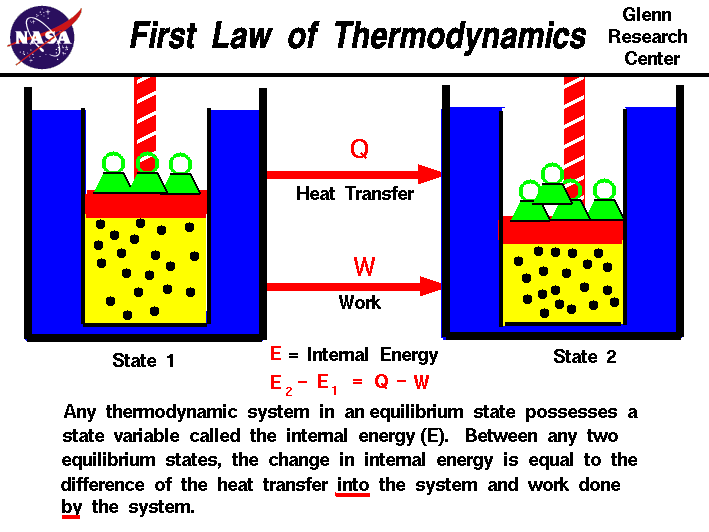
The first law of thermodynamics also known as the law of conservation of energy. First law of thermodynamics. When energy moves into or out of a system the system s internal energy changes in accordance with the law of conservation of mass. The most important and critical aspect of life revolves around the idea of energy.
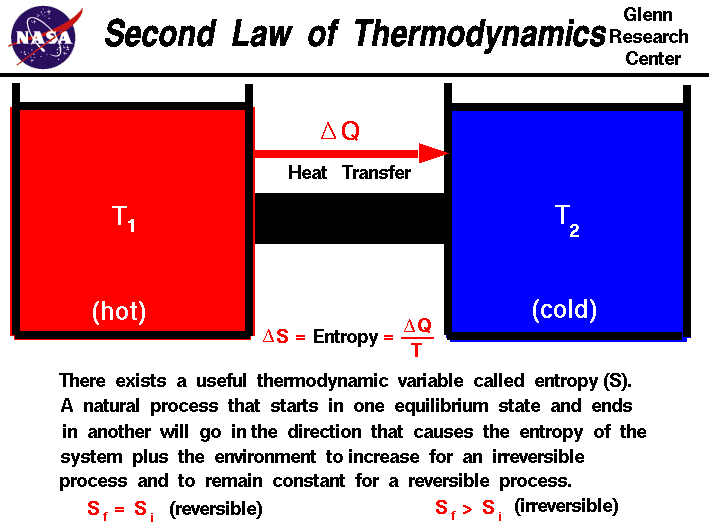
The state of the entropy of the entire universe as an isolated system will always increase. The first law of thermodynamics states that energy can be converted from one form to another but cannot be created or destroyed. Energy can only be transferred or changed from one form to another. The law is also known as the law of conservation of energy which states energy can transform from one form into another but can neither be created nor destroyed within an isolated system perpetual motion machines of the first kind are impossible according.
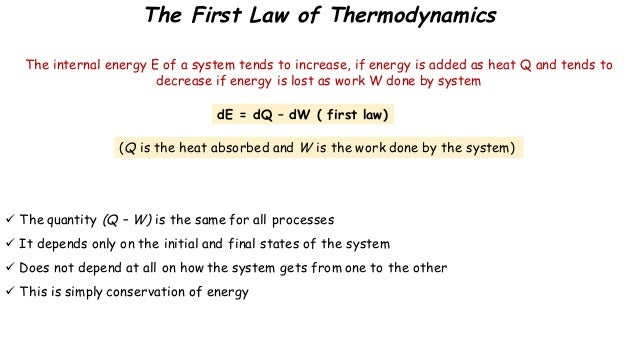
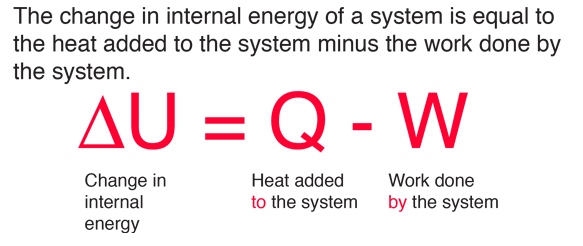
Statement of first law of thermodynamics. The first law of thermodynamics states that energy can be converted from one form to another with the interaction of heat work and internal energy but it cannot be created nor destroyed under any circumstances mathematically this is represented as. In other words the total energy of the universe remains constant. Energy can be.
The laws of thermodynamics are deceptively simple to state but they are far reaching in their consequences. However it is electrical energy that is converted. There is no transfer of matter into or out of the. The first law of thermodynamics also known as law of conservation of energy states that energy can neither be created nor destroyed.
The law of conservation of energy states that the total energy of an isolated system is constant. For example turning on a light would seem to produce energy. The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic processes distinguishing two kinds of transfer of energy as heat and as thermodynamic work and relating them to a function of a body s state called internal energy. Thermodynamics is the study of heat energy and other types of energy such as work and the various ways energy is transferred within chemical systems.
The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic systems in general the law of conservation of energy states that the total energy of an isolated system is constant. Wp ad camp 1 energy can neither be created nor destroyed but only be changed from one form to another form.







/GettyImages-954286708-388d95e8561449f6b78967110eec70ba.jpg)